The cellranger mkfastq pipeline is deprecated and will be removed in a future release. Please use Illumina’s BCL Convert to generate Cell Ranger-compatible FASTQ files. For detailed guidance, refer to the Generating FASTQs page.
Cell Ranger is a set of analysis pipelines that process Chromium Next GEM single cell data to align reads, generate feature-barcode matrices, perform clustering and other secondary analysis, and more (see list of example workflows and supported libraries). Cell Ranger includes five pipelines relevant to the 3' Single Cell Gene Expression, 5' Immune Profiling, and Flex assays:
-
cellranger count takes FASTQ files and performs alignment, filtering, barcode counting, and UMI counting. It uses the Chromium cellular barcodes to generate feature-barcode matrices, determine clusters, and perform gene expression analysis. The
countpipeline can take input from multiple sequencing runs on the same GEM well.cellranger countalso processes Feature Barcode data alongside Gene Expression reads. -
cellranger multi is used to analyze 3' and 5' Gene Expression, V(D)J, Cell Multiplexing, Flex, and Antigen Capture (BEAM) data. It takes FASTQ files from a combination of Gene Expression, Feature Barcode, and V(D)J libraries generated from a single GEM well.
cellranger multiperforms alignment, filtering, barcode counting, and UMI counting. It uses the cell barcodes to generate feature-barcode matrices, determine clusters, perform gene expression analysis, and provide preliminary cell type annotations. It is the recommended pipeline for analyzing a combination of 5' Gene Expression and V(D)J libraries (with or without Feature Barcode libraries) sequenced from the same sample. It is the only available pipeline for analyzing 3' Cell Multiplexing, Flex, and 5' Antigen Capture (BEAM) data. -
cellranger vdj takes FASTQ files for V(D)J libraries and performs sequence assembly and paired clonotype calling. It uses the Chromium cellular barcodes and UMIs to assemble V(D)J transcripts per cell. Clonotypes and CDR3 sequences are output as a
.vloupefile which can be loaded into Loupe V(D)J Browser. -
cellranger aggr aggregates outputs from multiple runs of
cellranger count,cellranger vdj, orcellranger multi, normalizing those runs to the same sequencing depth and then recomputing the feature-barcode matrices and analysis on the combined data. Theaggrpipeline can be used to combine data from multiple samples into an experiment-wide feature-barcode matrix and analysis. -
cellranger reanalyze takes feature-barcode matrices produced by
cellranger count,cellranger multi, orcellranger aggrand reruns the dimensionality reduction, clustering, and gene expression algorithms using tunable parameter settings. -
cellranger annotate takes the
molecule_info.h5and an optional.cloupefile produced bycellranger count,cellranger multi, orcellranger aggrto generate cell type annotations. This method assigns cell types by comparing each cell's gene expression profile to annotated reference datasets, providing a starting point for annotating your samples.
-
Run Cell Ranger on 10x Genomics Cloud Analysis
Skip Cell Ranger download and installation and get started with 10x Genomics Cloud Analysis, our recommended method for running Cell Ranger pipelines for most new customers. Use your web browser to easily generate Cell Ranger outputs from your FASTQ files and aggregate outputs from multiple runs, free for every 10x Genomics sample. Sign up for a free account or view tutorials and learn more.
-
Install and run Cell Ranger on your own computing infrastructure
Learn how to install and run Cell Ranger.
If you are beginning with raw base call (BCL) files, please use one of Illumina's demultiplexing software to demultiplex the BCL files for each flow cell directory. If you are beginning with FASTQ files that have already been demultiplexed, you can skip the demultiplexing step and begin with cellranger count. Please see the Specifying Input FASTQ pages for guidelines on which arguments to use for your scenario.
The exact steps of the workflow vary depending on how many samples, GEM wells, and flow cells you have, and whether you are including data from Feature Barcode, Cell Multiplexing, or Flex kits. This section describes a few possible workflows.
Watch a webinar on getting started with single cell data analysis.
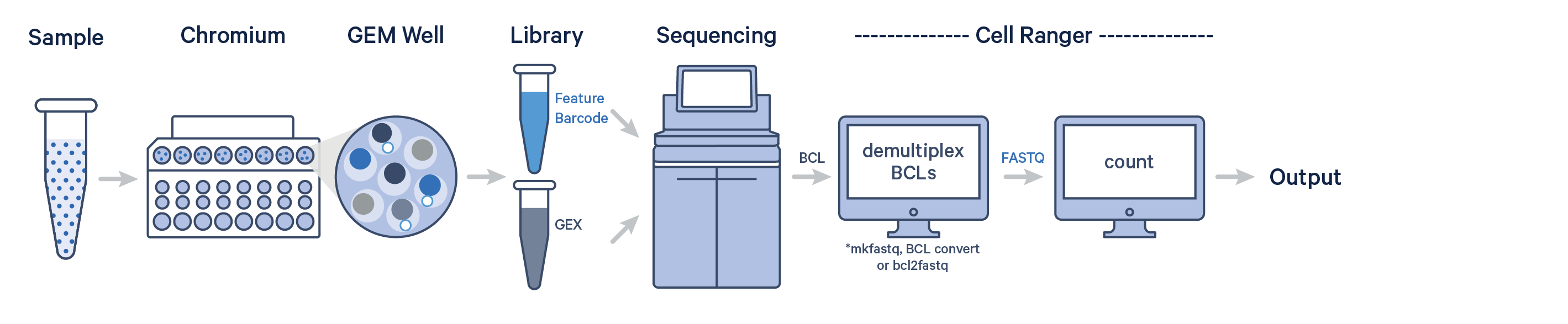
In this example, one sample is processed through one GEM well and sequenced on one flow cell. In this case, generate FASTQs using Illumina's demultiplexing software and run cellranger count as described in Single-Sample Analysis.
This example also illustrates two sequencing libraries. A single GEM well can yield multiple physical libraries: one Gene Expression library and one or more Feature Barcode libraries.
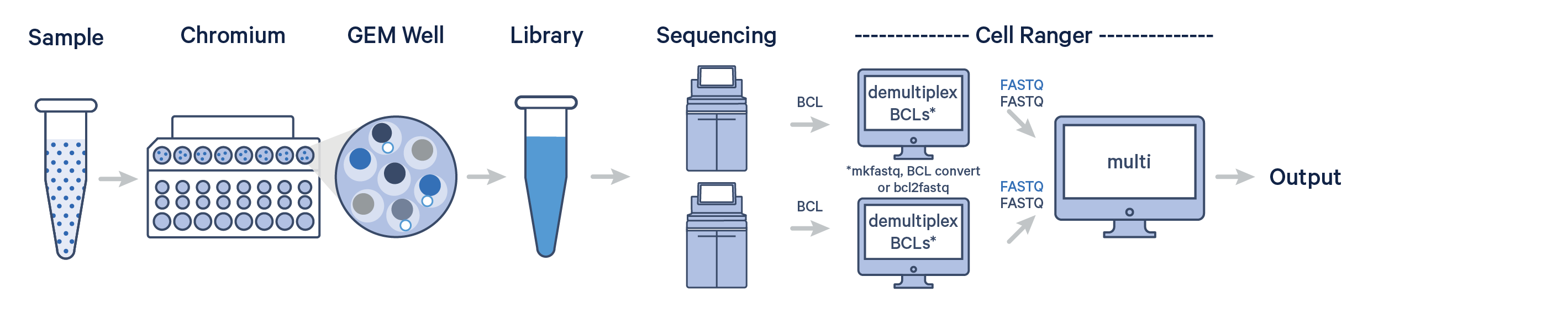
In this example, one sample is processed through one GEM well, resulting in one library which is sequenced across multiple flow cells. This workflow is commonly performed to increase sequencing depth. In this case, all reads can be combined in a single instance of the cellranger count or multi pipeline. This process is described in the Specifying Input FASTQ pages.
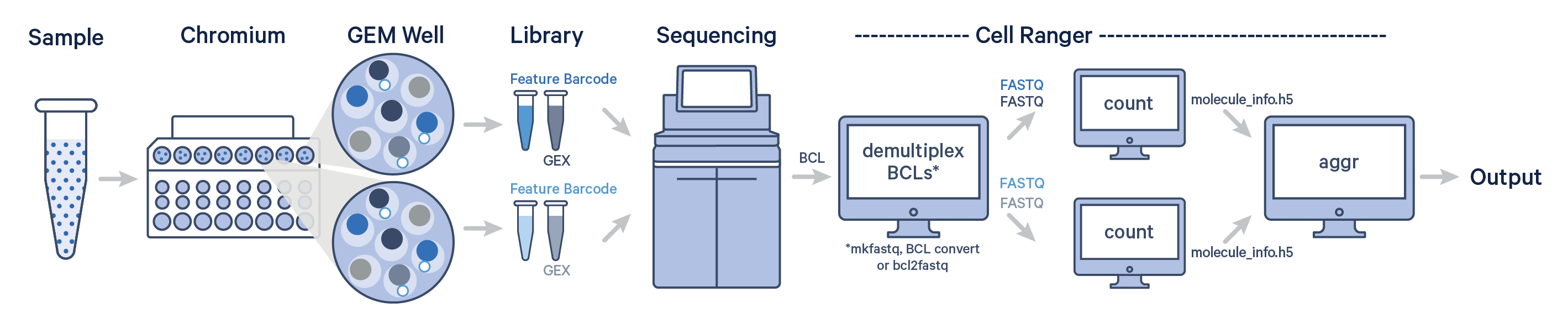
Here, one sample is processed through multiple GEM wells. This is typically done when conducting technical replicate experiments. The libraries from the GEM wells are then pooled onto one flow cell and sequenced. In this case, demultiplex the data from the sequencing run with one of Illumina's demultiplexing software, then run the libraries from each GEM well through a separate instance of cellranger count. Then you can perform a combined analysis using cellranger aggr, as described in Multi-Library Aggregation.
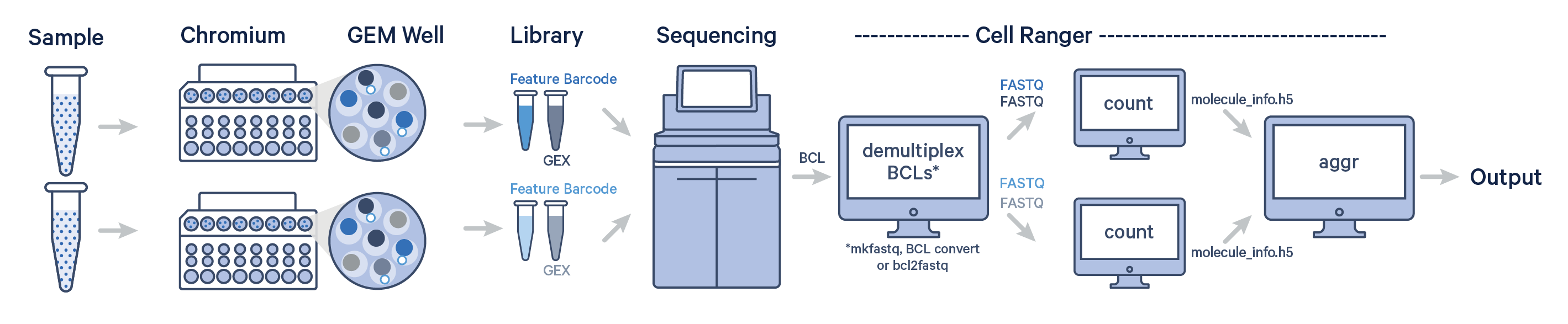
In this example, multiple samples are processed through multiple GEM wells, which generate multiple libraries and are pooled onto one flow cell. After demultiplexing, you must run cellranger count separately for each GEM well; if you have two GEM wells, then run cellranger count twice. Then you can aggregate them with a single instance of cellranger aggr, as described in Multi-Library Aggregation.
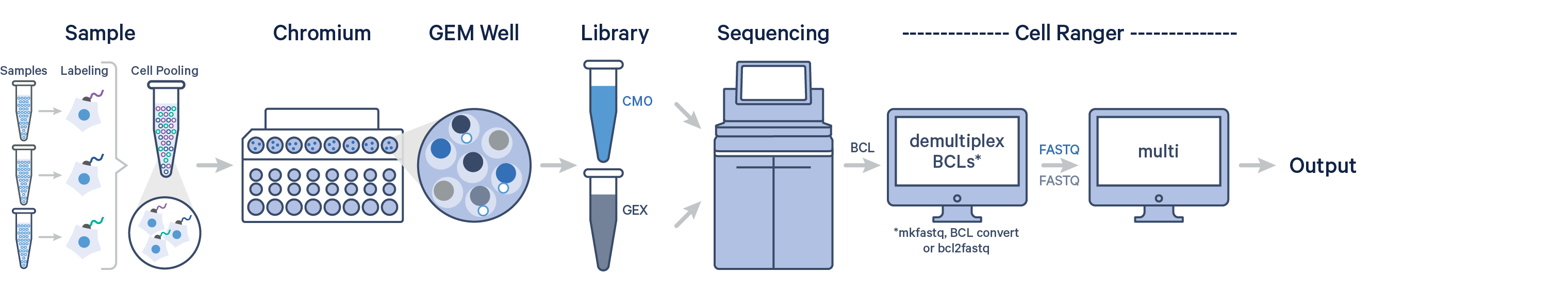
Cell Ranger 6.0 introduces support for analyzing Cell Multiplexing data. In this case, multiple samples are uniquely tagged with Cell Multiplexing Oligos (CMOs), enabling multiple samples to be pooled in a single GEM well. This results in a CMO and Gene Expression (GEX) library for each GEM well. After generating your FASTQ files, run the cellranger multi pipeline on the combined FASTQ data for the GEX and CMO libraries.
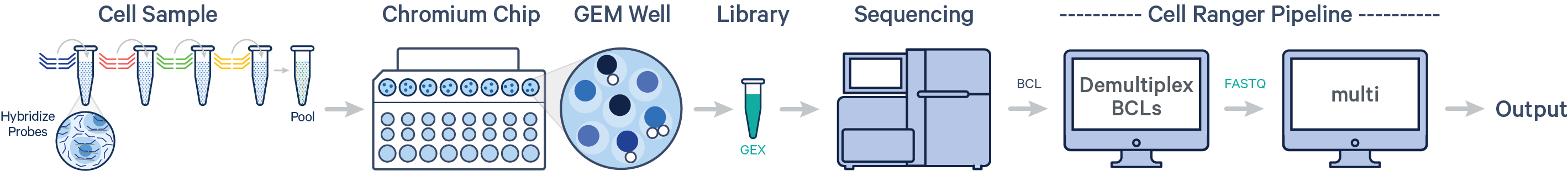
Cell Ranger 7.0 introduces support for analyzing Flex Gene Expression data. In this case, multiple samples are uniquely tagged with Probe Barcodes, enabling samples to be pooled in a single GEM well, resulting in one Gene Expression library. After generating FASTQ files, run the cellranger multi pipeline.