Innovations in single cell and spatial transcriptomics: Highlights from the 10x Genomics Symposium in Edinburgh
Edinburgh, November 7, 2024 - 10x Genomics Single Cell & Spatial Discovery Symposium events are hubs of innovation, bringing together researchers from different fields to share how they use single cell and spatial technologies to address real-world challenges. These events foster collaboration and offer an excellent opportunity to exchange ideas and gain fresh insights, regardless of one's expertise level.
The recent symposium in Edinburgh, Scotland, showcased remarkable advancements since last March’s Berlin event. Attendees reflected on the rapid progress in single cell and spatial transcriptomics while watching presentations on groundbreaking research, innovative tools, and new applications.
This article highlights key takeaways from the Edinburgh 2024 10x Genomics Single Cell & Spatial Discovery Symposium, featuring insights from customer speakers and new product developments:
- Decoding liver regeneration: Insights from single nuclei and spatial profiling
- Scaling spatial genomics using Xenium
- Creating a wound atlas: Advancing skin disease management in Atlantic salmon
- Optimizing Visium HD for human eye tissue
- Single nuclei sequencing and spatial transcriptomics to better understand multiple sclerosis
- Understanding the structural heterogeneity of lung adenocarcinoma
- New products
Highlights from featured presentations
Decoding liver regeneration: Insights from single nuclei and spatial profiling
The challenge
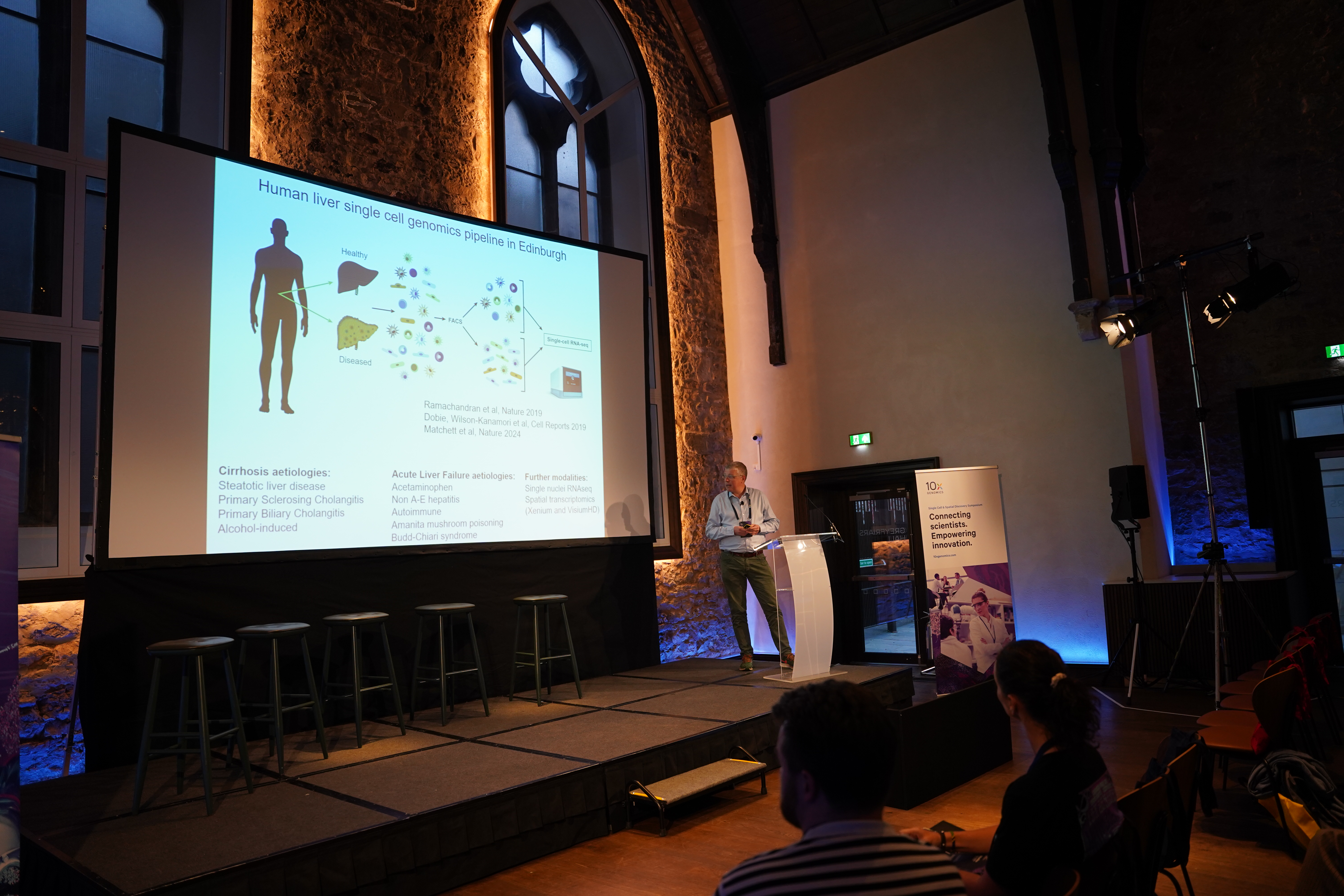
Dr. Neil Henderson, Professor of Tissue Repair and Regeneration, focuses his research on liver regeneration, particularly in Acute Liver Failure (ALF), a life-threatening condition with a 30% mortality rate. Due to the limited availability of donor livers for transplants, his team aims to develop pro-regenerative therapies to reduce the need for transplants.
Key findings
Analyzing diseased liver tissues, they observed that ALF samples showed a higher rate of proliferating hepatocytes compared to other diseases, suggesting active regeneration. Most proliferating cells were concentrated near necrotic tissue, the primary site of regeneration.
Using the 10x Single Cell 3’ Gene Expression assay (recently renamed to Universal 3’ Gene Expression), Dr. Henderson’s team profiled single nuclei from healthy and diseased frozen liver tissues collected during transplant procedures. Collaborating with the National Transplant Unit in Edinburgh, the US Acute Liver Failure Group, and other centers, they generated a high-quality multi-lineage gene expression dataset. They identified a specific hepatocyte subtype marked by ANXA2+ expression, later confirmed with single-molecule fluorescence in situ hybridization and immunofluorescence.
Their results revealed that liver regeneration involves more than hepatocyte proliferation. Hepatocytes near necrotic zones adopt a motile phenotype, contributing to wound closure before significant proliferation begins. In a mouse model of ALF, motile hepatocytes appeared within 36 hours of injury and faded after 42 hours. Advanced 3D live imaging confirmed these cells actively move to close damaged areas, a precursor to proliferation.
Therapeutic implications
Dr. Henderson’s work highlights a novel subpopulation of motile hepatocytes that mediate wound closure, offering potential for therapies that promote hepatocyte migration. “The liver prioritizes rapid wound closure to protect the gut-liver barrier, preventing systemic infections,” Dr. Henderson explained.
You can find more details about this work here.
Scaling spatial genomics for broad applications using Xenium
The challenge
Dr. Kenny Roberts, Senior Staff Scientist at the Wellcome Sanger Institute, is part of the institute’s High Throughput Spatial Genomics initiative. The team were the first in Europe to run Xenium in March 2023, initially choosing it as an automated platform with wide applicability as an update to their manual in situ sequencing platform. Owing to the excellent data Xenium has allowed them to generate across many projects, they have now scaled up their throughput with four instruments. To date, they have processed over 250 slides, assaying 55 million cells across 750 samples.
The new applications
Dr. Roberts explained a selection of his many collaborative projects, which span a broad range of different and new applications for Xenium:
Cystic fibrosis lung study
In collaboration with Dr. Josie Bryant, group leader at the Sanger Institute and expert in the lung microbiome, they have been pioneering the use of Xenium in mapping of bacterial gene expression within the respiratory system. Using lung tissue from cystic fibrosis patients, and a combined Xenium panel for the human lung plus ~50 bacterial genes, they successfully detected bacteria in the tissue and identified bacterial niches in the airways.
Marmoset brain study
In a collaboration with Dr. Jerry Chen at Boston University and Dr. Fani Memi at the Sanger Institute, the aim of the study was to map the transcriptomic profile of neurons in marmoset brains responsible for facial recognition processing. They used a custom 100-plex Xenium panel in addition to the Visium Spatial platform to profile marmoset brain tissue with both high-resolution imaging and full transcriptome sequencing-based approaches.
Cultured microglial cells
Working with Dr. Aleksander Gontarczyk in the laboratory of Dr. Andrew Bassett, the team first experimented with coatings for the Xenium slides that would support the growth of microglia cells derived from induced pluripotent stem cells (iPSCs). The cells were grown directly on the slides and then fixed and permeabilised. Using a 380-plex immune-oncology panel, they captured the spatial gene expression of these microglia cells.
Using the same cells they also launched a project to detect single nucleotide polymorphisms (SNPs). For this, the team collaborated with 10x Genomics to create a custom 100-plex panel, which included 52 gene expression targets and 48 SNP-specific probes targeting 24 SNPs. The SNP probes were carefully designed to distinguish between single nucleotide differences by positioning the probe junction at the variant position. Ultimately, 21 out of 24 SNP probes provided reliable results, allowing them to map SNPs accurately across the cell population. This SNP detection could be used in the future in pooled optical screens to distinguish between different donors and, in tissue samples, to identify and track tumor clonality.
Adult heart tissue
Adult heart tissue contains a diverse range of cells, from small immune cells to large cardiomyocytes, making cell segmentation particularly challenging. Prior to the release of the Xenium multimodal cell segmentation solution, the team used an immunofluorescence staining strategy to augment cell segmentation. After assaying the tissue using a 377-plex multi-tissue Xenium panel, they stained the same sections with a panel of three antibodies to mark cells and structures of interest. This approach enabled them to mark protein expression and outline the different cell types effectively.
Future implications
Dr. Roberts highlighted that “By pushing Xenium’s capabilities, we are creating a roadmap for future applications, with the potential to redefine how we study complex tissue ecosystems across species.” His team’s work demonstrates how the Xenium can support diverse applications, unlocking new insights into cell biology, disease, and tumor genetics.

Creating a wound atlas: Advancing skin disease management in Atlantic salmon
The challenge
Dr. Ruiz Daniels, a lecturer in Aquaculture Genomics at the University of Stirling, is leading the creation of a Wound Atlas of the Skin for Atlantic salmon. This project aims to address skin diseases, like sea lice infestations, which are a major challenge in aquaculture. Her research focuses on understanding normal and abnormal wound healing in salmon and investigating why some wounds fail to heal, with the goal of improving fish health and management practices.
Key findings
Dr. Daniels’ team optimized a nuclei extraction protocol for salmon skin to enable single nuclei analysis using 10x Single Cell 3’ Gene Expression assay (now Universal 3’ Gene Expression). To explore why other salmonid species resist sea lice better than Atlantic salmon, they conducted single nuclei RNA sequencing across four species at multiple stages of infection. They found that keratinocytes drive epithelialization during wound healing, while mesenchymal stromal cells (MSCs) and immune cells also play key roles in the process.
Using Visium on cryopreserved salmon skin, a challenging process, they identified distinct niches of MSC subtypes, consistent with their single nuclei data. Since salmon lack bone marrow, these MSC subtypes seem to take on regenerative roles that are essential for the remodeling and repair of skin to maintain its functional integrity. One major challenge was accurately labeling cells, as few established markers exist for salmon. The team has relied on markers conserved across species and plans to use Xenium to further refine their understanding of these cellular processes.
Implications for aquaculture
Finding specific markers could revolutionize disease management in salmon aquaculture, reducing costs worldwide and improving fish health and resilience.
You can find more details about this work here and here.

Optimizing Visium HD for human eye tissue
The challenge
Dr. Pietro Maria Bertelli, a Research Fellow at Queen’s University Belfast, studies gene expression in the human eye, focusing on pseudoxanthoma elasticum (PXE). PXE, caused by ABCC6 gene mutations, leads to abnormal calcification in the eye’s elastic layers, disrupting nutrient transport and causing retinal damage.
Dr. Bertelli aimed to use the 10x Genomics Visium HD Spatial Gene Expression (Visium HD) platform to analyze retinal changes in donors’ fresh fixed samples. The goal was to identify potential therapeutic targets in specific retinal regions. However, challenges occurred due to existing protocols not being optimized for human eye tissue.
The large size of the human eye (23.5-25mm) created a challenge, as the Visium HD capture area is just 6.5 x 6.5 mm. This forced the team to focus on specific regions of interest rather than full-eye sections. Another challenge was adhesion—internal air content made it difficult to keep tissue attached to slides. Adjustments to incubation times improved adhesion, but did not fully resolve detachment issues.
Although RNA quality was not ideal, the DV200 quality metrics were encouraging in some cases, so the team decided to continue with the experiment. Though the final data fell short for analysis, the experience provided valuable learnings to refine future experiments and further optimize the Visium HD workflow.
Future implications
Reflecting on his experience, Dr. Bertelli emphasized the need to refine spatial transcriptomics protocol for human eye tissue. These high resolution techniques could offer new insights into PXE-related retinal damage and help in identifying early markers of calcification, ultimately advancing diagnostics and treatment for PXE.

Single nuclei sequencing and spatial transcriptomics to better understand multiple sclerosis
The challenge
Multiple sclerosis (MS) affects the central nervous system, leading to demyelination and neurodegeneration in gray matter, which controls muscle function and sensory perception. Kellie Horan, PhD student at the University of Edinburgh, is exploring early molecular changes to uncover pathways for potential neuroprotective therapies.
Using a mouse model of MS, Horan used lysolecithin to induce myelin damage. She analyzed brain samples using 10x Single Cell 3’ Gene Expression assay (nuclei), Xenium, and Visium HD to examine gray matter cell types, especially parvalbumin (PV)-positive neurons, key regulators of brain activity that are highly vulnerable in MS.
Key findings
Initial single nuclei RNA sequencing revealed minimal differences in PV-positive neurons between diseased and control samples, with just four genes altered, some of which are linked to Alzheimer’s pathways. Suspecting dilution effects from gray matter’s layered structure, Horan’s team reanalyzed samples by focusing on specific layers. This approach revealed more distinct disease-specific changes.
To refine her findings, Horan turned to spatial transcriptomics. Using paraffin-embedded brain tissue, she identified lesion areas with immunofluorescence, then studied gene expression changes with Xenium using the 248-plex mouse brain panel and Visium HD. This provided a clearer picture of transcriptomic changes in affected neurons.
“Spatial transcriptomics is a powerful tool for studying a complex disease like MS, but it is important to benchmark technologies and choose the best one for your question,” Kellie Horan noted.
Future implications
Her work highlights the value of spatial approaches in identifying early molecular changes in gray matter, which could inform future biomarkers or therapies for MS.

Understanding the structural heterogeneity of lung adenocarcinoma
The challenge
Lung cancer remains a leading cause of cancer-related deaths in the UK, with non-small cell lung cancer (NSCLC) accounting for 80-85% of cases.
Dr. Nicolas Poulain, a Postdoctoral Research Scientist at the CRUK Scotland Institute, studies lung adenocarcinoma, a subtype of NSCLC. His research focuses on understanding the diverse tissue structures (morphologies) within and across tumors. These variations highlight the complexity of tumor growth and progression.
Key findings
Using the Xenium platform with a custom 468-plex gene panel, Dr. Poulain analyzed tissue microarrays (TMAs) from 40 patients. Each TMA contained up to three spots per patient, enabling precise mapping of gene expression patterns tied to different tumor morphologies. For analysis, he applied tools like inSituType for cell typing, Harmony for batch correction, and Leiden clustering.
The results revealed significant heterogeneity within tumors, showing that different tumor areas can have unique cellular compositions and growth behaviors. Certain regions showed cellular “plasticity,” particularly in solid sub-regions. Tumor-edge cells, for instance, had higher Ki67 expression when compared to the tumor center, a marker for proliferation, suggesting these cells drive tumor expansion.
Therapeutic implications
This research underscores the importance of spatial heterogeneity in lung adenocarcinoma. By identifying how different tumor regions contribute to growth and aggressiveness, Dr. Poulain’s work leads the way for more personalized therapies targeting distinct cell populations. “By dissecting these spatial characteristics, we can better understand tumor progression and potentially discover new therapeutic targets,” he explained.

New products
Chromium Single Cell
At the Berlin symposium last March, attendees were excited about the upcoming GEM-X product line. Key features like preventing clogs, improving cell capture efficiency, and reducing sequencing costs are now a reality. As Sarah Taylor, Senior Director Applications at 10x Genomics, explained, GEM-X includes redesigned microfluidics chips and enhanced kit chemistry, boosting cell recovery rates (65% to 80%) and sensitivity while keeping assay costs stable and lowering cost per cell.
GEM-X powers two of the three Chromium Single Cell product families: the Universal and Flex Gene Expression. The Epi Chromatin product family that includes single cell ATAC and multiome capabilities, remains based on Next-GEM technology.
The Universal Gene Expression product family comprises the Universal Gene Expression 3’ and 5’ assays for studying polyadenylated RNA across all species. A new on-chip multiplexing feature allows pooling samples directly on the chip without tagging or hashing, and is now available for pre-order.
Flex Gene Expression, designed for fixed samples, also now utilizes GEM-X technology. It offers improved cell recovery, requires fewer input cells, and supports multiomic approaches, enabling the detection of both extracellular and intracellular proteins.
10x Genomics has also launched the Chromium Xo, a budget-friendly addition to the Chromium X series, offering more flexibility to meet researchers' needs.
Spatial transcriptomics
In spatial transcriptomics, Visium HD and Xenium are now delivering impactful results. Visium HD provides high resolution, supports fresh and fixed tissue workflows, and will soon include a 3’ RT-based assay for polyadenylated mRNA. Xenium provides predesigned panels, such as a 5,000-plex panel, along with customizable options for applications like xenografts, bacterial, or viral genes. Protein panels and software upgrades are on the horizon.
Conclusion
The 2024 10x Genomics Symposium in Edinburgh showcased the transformative impact of their technologies, inspiring researchers to push the boundaries of single cell and spatial biology. From breakthroughs in liver regeneration and aquaculture health to insights into neurodegeneration and cancer, the event was a hub for innovation and collaboration, connecting experts across disciplines.
Looking ahead, 10x Genomics is dedicated to advancing technology with a clear philosophy centered on three pillars: expanding biological insights through broader applications, higher throughput, and greater resolution; ensuring user-friendly solutions; and reducing costs to make cutting-edge science more accessible. Guided by this vision, the future of single cell and spatial biology is filled with exciting new possibilities.

This blog was written by guest author Cátia Moutinho, PhD. She is a scientific consultant and the founder of The Single-Cell World, a digital community of scientists dedicated to helping researchers understand all things single cell technology. You can find more of her blog articles here.
