Single cell techniques enable progress in making islet transplantation a reality for more people with diabetes
In honor of National Diabetes Awareness Month, we highlight a promising development in treatment options for patients with type 1 diabetes. Beyond drugs that manage the symptoms of diabetes, patients may consider pancreatic islet transplantation as an experimental treatment to restore crucial insulin-secreting beta cells, but transplants can be rejected by the body and donors are limited. Now, with the help of single cell analysis, scientists have discovered a key signaling pathway that induces successful differentiation of stem cells into functional beta-like cells, expanding the options for patients with diabetes.
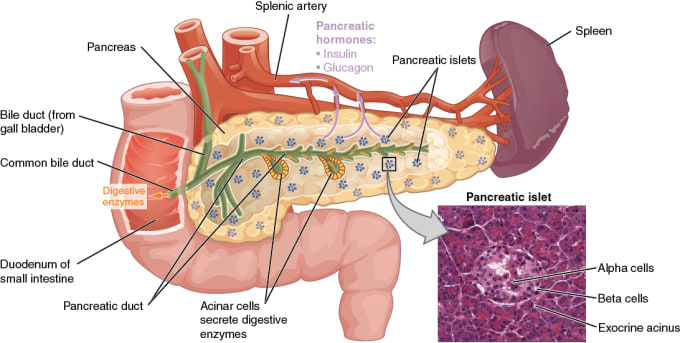
In diabetes, the body has trouble making or using insulin, so any glucose that is ingested through eating and drinking stays in the blood and does not reach cells. In type 1 diabetes, the immune system attacks and destroys pancreatic islet beta cells, which make insulin. In type 2 diabetes, the body no longer responds efficiently to insulin, so the beta cells become overworked and exhausted, leading to a decrease in the number or volume of cells. While there are treatments for the symptoms of diabetes, there is currently no diabetes drug on the market that can restore beta cells to produce functional levels of insulin.
Pancreatic islet transplantation seems like an ideal therapy for people with diabetes, but so far, it remains an experimental treatment (for type 1 diabetes only) and comes with significant risks. These include developing autoimmunity to the donor islet cells, which come from the pancreas of a deceased organ donor. One of the major challenges to treating more type 1 diabetics with islet transplantation is the shortage of donor tissue, which has led scientists to search for an alternative source of these cells. In the last few years, this has become an important field of study as researchers continue to conduct research looking at the potential of using stem cells to generate islet cells that could repair or replace damaged tissue.
While the jury is still out on just which cells will ultimately be best, a variety of types of stem cells are being investigated to replace beta cells, including human embryonic stem cells (hESCs), induced pluripotent stem cells (iPSCs), and adult progenitor cells. All have advantages and disadvantages to being used to derive beta or beta-like cells, including each one’s different capacity to differentiate into beta cells, ability to maintain functionality in diabetic patients, and propensity to elicit an autoimmune reaction.
Previous studies have shown promise in successfully inducing differentiation of hESCs and iPSCs into beta-like cells, but more research is needed to ferret out exactly which signaling pathways are involved in creating functional beta-like cells that can be used as therapy. In a previous study out of Dr. Eiji Yoshihara’s lab at the Salk Institute for Biological Studies in California, he and his team discovered that estrogen-related receptor γ (ERRγ) expression plays a key role in the maturation of functional beta cells that can produce insulin in response to glucose, and they described an approach for deriving beta-like cells from iPSCs in vitro (1) by overexpressing ERRγ.
Building on this work, scientists led by Dr. Yoshihara and Dr. Ronald Evans (at the Salk Institute) described a protocol by which they were able to drive the differentiation of iPSCs into insulin-positive, glucose-responsive beta-like cells (2). Using 3D cultures to mimic the cellular architecture and diversity of the islet cell environment, they generated human islet-like organoids (HILOs) that, when transplanted into the kidney, were able to restore glucose homeostasis in immunodeficient diabetic mouse models for 40 days—and which displayed similar efficacy to human islet transplantations.
Previous studies have shown that WNT4 expression is increased during the functional maturation of mouse islets, and that the WNT pathway leads to β-cell maturation and an increase in glucose-stimulated insulin secretion in human islets. In this work, they used single cell RNA-sequencing to find that treating the HILOs with WNT4 helped drive transcriptional changes that led to metabolically mature and, therefore, functional HILOs. To identify changes in chromatin accessibility resulting from WNT4 treatment, they used assay for transposase-accessible chromatin using sequencing (ATAC-seq) to discover 123 genes with increases in expression and chromatin accessibility, including metabolic pathway, β-cell maturation factor, and ERRγ-target genes.
In an effort to alleviate immune rejection, a dangerous pitfall of islet transplantation, the team created HILOs both with and without PD-L1-expressing genes, hypothesizing that PD-L1, an immune checkpoint protein, could provide immune protection for the transplanted cells. After transplanting the HILOs into diabetic mice, they found that only PD-L1+ HILOs maintained glucose homeostasis for any length of time (more than 50 days). Using FACS-based immune profiling of recovered grafts, they noted reduced numbers of CD45+ immune cells, including T cells and natural killer cells, in PD-L1+ grafts, suggesting PD-L1 can protect the HILOs against an autoimmune reaction. This finding points to the possibility of developing islet transplantation treatments that no longer require intensive immune-suppressive drugs.
Hear from the scientists who conducted this study, and find an FAQ about the new therapeutic possibilities their findings open up for people with diabetes, in this article from the Salk Institute.
Advancing stem cell therapies for type 1 diabetes
This work adds to an increasing number of studies inching closer to defining a promising alternative to standard therapies or those derived from organ donor cells in the treatment of diabetes, particularly type 1 diabetes. The ability to generate glucose-responsive human beta (or beta-like) cells that can be used in islet cell transplantation can potentially transform diabetic patients’ lives, replacing cells that no longer produce insulin and addressing the root cause of this disease.
References:
- E Yoshihara et al., ERRγ is required for the metabolic maturation of therapeutically functional glucose-responsive β cells. Cell Metab. 23, 622–634 (2016).
- E Yoshihara et al., Immune-evasive human islet-like organoids ameliorate diabetes. Nature. 586 (7830), 606–611 (2020).
