Booster shots & breakthrough infections: How T cells respond to repeated SARS-CoV-2 exposure
Though COVID-19 rates have dropped significantly since the omicron surge in early 2022, a new trend is emerging. Where once we asked friends and family if they’d gotten COVID yet, now it’s not unreasonable to ask how many times they’ve been infected. Twice? Three times? Breakthrough cases—meaning SARS-CoV-2 infections in individuals who have been fully vaccinated—are the new concern in the pandemic landscape of viral variants and vaccines. This has left researchers with an essential challenge: how to optimize vaccination strategies for better long-term immunity.
The question comes down to how our immune systems are impacted by initial and repeated exposure to SARS-CoV-2 antigens, either in the form of natural infection, primary vaccination, or booster shots. This is still murky, as it likely depends on the order of immunization events in individuals (infection first, then vaccination, or vice versa), variation in the exact SARS-CoV-2 antigen, and, fundamentally, the body’s mechanisms to develop immunological memory.
[Jump to Minervina et al. findings]
T cells at the ready
Memory T cells are at the core of these mechanisms. In an ongoing infection, or when a pathogen is cleared from the body, a subset of antigen-specific CD8+ T cells will differentiate into central memory, effector memory, or resident memory T cells (1, 2). Activation state and location of residence within the body are the key distinctions between these cell subtypes. For example, central memory cells reside in the lymph nodes or bone marrow and maintain high proliferative potential, while resident memory cells linger in an activated state among epithelial cells of previously infected organs (1). However, each of these long-living T-cell subtypes shares a function in providing surveillance to protect the body from another encounter with the same pathogen. Surveillance is powered by T-cell receptors (TCRs) that recognize antigen peptides presented on the surface of other cells by the major histocompatibility complex (MHC; 3).
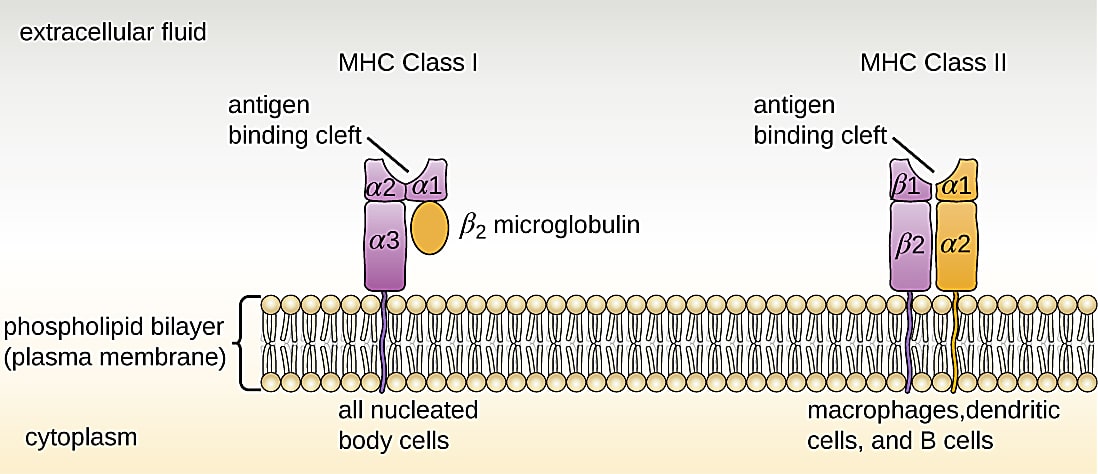
However, when it comes to multiple encounters with SARS-CoV-2, either through reinfection or vaccination, Minervina et al. note:
“It is largely unknown if repeated exposure to the same SARS-CoV-2 antigens boosts preexisting T cell memory and, further, if an exposure to a novel antigen (for example, infection after vaccination or infection with a new viral variant) induces de novo memory and diversifies the T-cell receptor repertoire” (3).
Understanding how repeated exposure to SARS-CoV-2 antigens affects memory T-cell biology would be vital information to guide COVID-19 vaccination strategies; in particular, determining how effective current vaccine programs are to prime immunological memory and clarifying the benefits and optimal cadence of booster shots.
Concerns about repeated antigen exposure
Existing research on repeated antigen exposure suggests it can actually have a mixed outcome. Commenting on vaccine-induced T-cell memory in their book, How Vaccines Work, Claire-Anne Siegrist, MD, and Paul-Henri Lambert, MD, note that, “the establishment of T-cell memory requires some time after the initial priming. Secondary T-cell responses are lower if vaccine boosters are given too early” (1). In other words, shoot too fast, and you’ll miss the target of an optimal memory T-cell immune response.
More significantly, continuous exposure to antigens has been shown to cause T-cell exhaustion. Studies of chronic lymphocytic choriomeningitis virus (LCMV) have demonstrated a correlation between levels and persistence of antigen stimulation and severity of T-cell exhaustion (4). One study found that if CD8+ T cells were primed with a chronic strain of LCMV, but antigen was removed about one week after infection, those T cells could fully differentiate into functional memory T cells. However, if they were exposed to antigen continuously for multiple weeks, the T cells became exhausted and could not recover memory-like functionality even after removing antigen (4).
The cadence and persistence of antigen exposure isn’t the only concern. Which antigen primes the immune response also matters. SARS-CoV-2 spike antigens are the only component of current approved COVID-19 vaccines, but repeated, exclusive exposure to spike antigen could bias immune memory, leaving the door open to possible breakthrough infections by novel viral variants (3). Again, this makes it crucial that we understand how current vaccination and booster programs are influencing the dynamics of immune memory formation, and, particularly, if they can be optimized to promote TCR diversity and cross-reactivity to novel SARS-CoV-2 antigens.
Surveying T-cell responses after first, second, and third antigen exposure
In order to better understand how T-cell memory is affected by repeated SARS-CoV-2 antigen exposure, a team of researchers from St. Jude Children’s Research Hospital studied peripheral blood mononuclear cell (PBMC) samples from 55 adults representing four subgroups with unique antigen exposure combinations. This included: 1) a fully vaccinated group that remained SARS-CoV-2 negative over the duration of the study; 2) a group that tested positive and recovered from mild infection; 3) a group that was infected then received one dose of vaccine; and 4) a group that was infected then received two doses of vaccine. Additionally, the team took samples from donors that tested positive for SARS-CoV-2 after receiving both vaccine doses, the so-called “breakthrough” group (3).
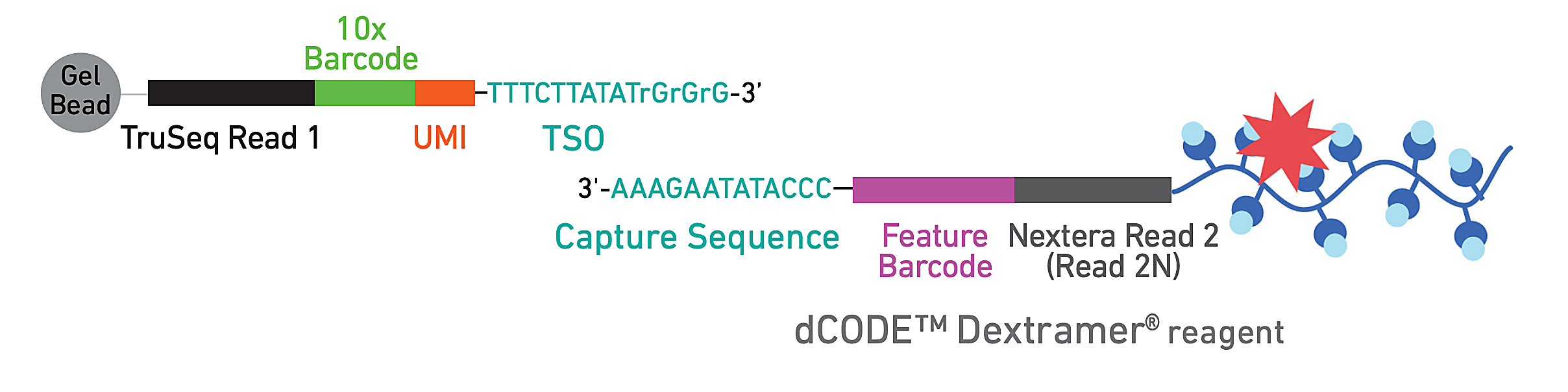
To evaluate epitope-specific CD8+ T cell responses, they developed a panel of 18 SARS-CoV-2 spike-derived and non-spike epitopes, including several similar orthologs from common cold coronaviruses (CCCoV). Donor PBMCs were subsequently stained with DNA-barcoded, fluorescently labeled dextramers bearing the epitope panel—which act like MHC structures presenting antigen to T cells—allowing the team to isolate epitope-specific T cells by FACS. Then they used single cell RNA-sequencing, T-cell receptor sequencing, and CITE-seq to generate comprehensive phenotypes of these reactive T cells.
Analysis of T-cell phenotypes showed that natural infection, breakthrough cases, and vaccination led to the formation of potent T-cell memory. Notably, there was a clear shift in memory T-cell composition between donors who were previously infected naturally then received either one or two vaccine shots. The additional vaccine, representing a third antigen encounter in these donors, appeared to drive a more highly differentiated effector memory phenotype among spike-specific T cells—though it remains unclear if this phenotype is associated with more efficient protection or durable memory (3).
Addressing the concern that additional exposures could narrow TCR repertoire diversity, the team actually found that the diversity of spike-specific and non-spike-specific repertories among all groups was comparable. A diverse non-spike-specific T-cell memory was seen even in breakthrough cases, suggesting the initial vaccination program did not bias immune memory to the spike protein exclusively. Additionally, analysis of the relative abundance of T-cell clones across donors revealed that five of the most abundant clonotypes recognized both SARS-CoV-2 spike epitope and CCCoV orthologs, further confirming the potential to induce a diverse and even cross-reactive T-cell memory pool.
The research team also observed an association between the presence of an exhausted T-cell cluster and other clusters experiencing clonal expansion and cell proliferation across all groups. This suggested donors were still in the active rather than memory state of the CD8+ T-cell response. Moreover, they observed the number of exhausted cells decrease over time from the point of antigen exposure, leading the team to conclude that while this subset is common in mild infections, it’s ultimately transient and does not drive pathology (3).
Maturing, not impairing, the T-cell response
The results from this comprehensive study give hope that there are likely no long-term negative effects of repeated SARS-CoV-2 antigen exposure on T-cell memory. Countering concerns about the potential for negative effects, it seems that repeated exposure has a maturing effect, inducing both greater T-cell effector function and diverse reactive potential to spike protein and other SARS-CoV-2 internal proteins. Individuals who experience breakthrough infection can also rest assured—infections are typically mild and their T-cell memory pool is sufficiently diverse to maintain immunity to possible SARS-CoV-2 variants.
In honor of National Immunization Awareness Month, we’d like to thank the scientists at St. Jude Children’s Research Hospital for spearheading this important study. And we thank the many other researchers and institutions leading the way in deepening our understanding of vaccine efficacy, COVID-19, and other infectious diseases.
Learn more about the single cell tools used for this study and explore compatible tools to analyze antigen specificity.
References:
- Siegrist CA and Lambert PH. Chapter 2: How Vaccines Work. The Vaccine Book (Second Edition) (2016).
- Hofmann M, et al. Editorial: Memory T cells in chronic infections and tumors. Front Immunol 12: 656010 (2021). doi: 10.3389/fimmu.2021.656010
- Minervina A, et al. SARS-CoV-2 antigen exposure history shapes phenotypes and specificity of memory CD8+ T cells. Nat Immunol 23: 781–790 (2022). doi: 10.1038/s41590-022-01184-4
- Wherry JE and Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 15: 486–499 (2015). doi: 10.1038/nri3862
