Enter the year of the rabbit (researcher)
The Lunar New Year—also known as the Chinese New Year—started on January 22. According to the Chinese Zodiac, each lunar year is represented by an animal, and people born in that year have characteristics similar to the designated animal. 2023 is the year of the rabbit. People born this year are purportedly calm, peaceful, and detail-oriented. In honor of the Year of the Rabbit, we are highlighting a paper that uses rabbits to model early development and uncover details of human embryonic development that the mouse just can’t.
Early human embryonic development is something of a black box. Researchers legally cannot study human embryos that have been developing for more than 14 days, the point at which an embryo individuates or cannot split into identical twins (though, in 2021, the International Society for Stem Cell Research suggested researchers seeking to study embryos beyond this time point be considered on a case-by-case basis) (1).
Most developmental biologists instead have turned to other models, primarily the mouse, to understand human embryonic development. But these models are less than ideal. Human’s closest relatives, non-human primates, are plagued by technical challenges, including expensive upkeep, long pregnancies, and few offspring. Mice are technically ideal given their short generation times and large litter sizes, but their development varies significantly from our own.
Researchers have started troubleshooting new models to investigate the mechanistic underpinnings of embryonic development. While some have begun crafting in vitro embryo models using stem cells (2), others have turned to a historically underused animal model: the rabbit.
Recently, a group of researchers published a preprint that details the analysis of rabbit embryos at various developmental stages using 10x Genomics Chromium Single Cell Gene Expression and Chromium Single Cell ATAC and produced the most detailed transcriptional and chromatin accessibility atlas of a non-rodent embryo to date (3). They compared their data to previously collected mouse data and identified both known and novel signaling interactions not found in mice. The authors think their data make a compelling case for the rabbit as a superior model for human embryonic development and hope their work inspires others to investigate novel models to shine a light on this black box.
“Our results highlight the utility of the rabbit as a model for a new wave of mammalian development research that will combine the power of traditional comparative embryology with state-of-the-art comparative single cell genomics approaches,” wrote the authors.
“When researching rabbit development for this manuscript, one of the most useful references turned out to be [from] 1905…It may have taken over 100 years, but it is now realistic to imagine a similar compilation of single cell genomics atlases for over a dozen vertebrates within the next few years.”
The last 100 years
A major challenge for developmental biologists is how early development differs across mammals, especially between rodent and non-rodent species. But there are some commonalities.
Development always starts when an egg is fertilized by a sperm and a zygote is formed. Cells within the fertilized zygote divide and divide and divide until they form a ball of undifferentiated cells known as a blastocyst (4). An outer layer of trophoblast cells—cells only capable of differentiating to become extraembryonic tissue—surround a mostly empty cavity with a tightly packed bundle of cells at the top known as the inner cell mass (ICM). Cells in the ICM go on to produce every other cell in the body, and are also the source of embryonic stem cells.
This is the point where mammalian development diverges across species—especially between mice and humans. In both mice and humans, the ICM cells form two layers: the epiblast and the hypoblast (4). In humans (and most amniotes), the epiblast lies flat above the hypoblast, forming a flat disc-shaped epiblast. But in mice, the epiblast lines the outer cells of the blastocyst and the hypoblast becomes the cellular border of the entire early embryonic structure, taking the appearance of an egg cylinder. Essentially, the mouse embryo is inverted compared to the human embryo at this stage. Early embryonic mouse development differs in other major ways, too, including during implantation, another developmental black box.
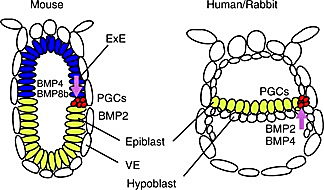
These differences are more than a headache for researchers; in pharmaceutical research, they can have major consequences during preclinical trials. 90% of drugs fail during clinical trials, and 50% of drugs that fail during phase II fail due to previously unknown toxicities (5). In some cases, a different animal model could have caught these toxicities before millions were spent on human trials. For example, physicians across Europe prescribed the drug thalidomide for morning sickness amongst pregnant women in the late 1950s and early 1960s. Due to thalidomide, 10,000 children across 46 countries were born with deformities, most often in their extremities (6).
The thalidomide tragedy spurred greater restrictions and higher standards for preclinical testing. Regulatory guidelines now require that embryo–fetal developmental toxicity testing be done in rodent and non-rodent species—the most common being the European rabbit, Oryctolagus cuniculus (7). For example, thalidomide causes phocomelia—severe malformation of extremities—in rabbits and humans, but not in mice.
Despite the potential for rabbits as a model of early human development, researchers haven’t done a deep, molecular analysis of their early development—until now.
The modern age
The researchers analyzed rabbit embryos at the implantation, gastrulation—when the three primary germ layers form—and early organogenesis stages, which correspond to gestational days (GD) 7, 8, and 9 of the New Zealand White Rabbit, respectively. They used our Chromium Single Cell Gene Expression kit to obtain high-quality single cell RNA-seq profiles of 13,647 cells from six individual GD7 embryos, 34,686 cells from two pooled and three individual GD8 embryos, and 97,773 cells from four GD9 embryos. The researchers implemented an algorithm to predict the type of each cell profiled based on a recent atlas of mouse gastrulation and early organogenesis (8). After some manual curation, they identified 67 cell types across their collection of 146,133 profiled cells.
The researchers then honed in on trophoblast differentiation. Trophoblasts go on to produce extraembryonic cells and tissue, most of which make up the placenta. How this differentiation process works, and how its dysregulation contributes to conditions such as preeclampsia, is yet another developmental black box.
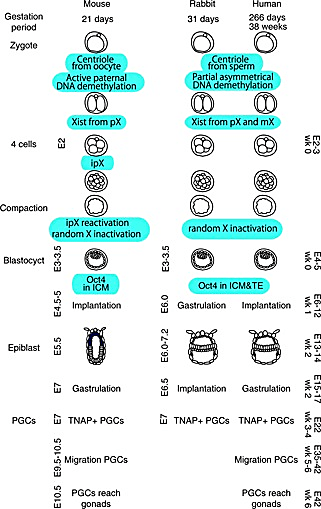
Trophoblast differentiation happens before gastrulation, when both human and mice embryos are very small. Once they expand, they are already buried deep in the uterine wall, making it difficult to isolate the trophoblasts without also grabbing maternal tissue. While gastrulation happens after implantation in mice and humans, implantation and gastrulation occur at the same time in rabbits so researchers can capture larger embryos preparing to undergo gastrulation before they implant in the uterine wall.
The researchers did find evidence of more, diverse extraembryonic cell types in their data than in the atlas of mouse gastrulation (8). They found expression of key markers of cytotrophoblasts and syncytiotrophoblast progenitors—precursors for the cells that initiate implantation in the uterine lining—that were not present in the mouse atlas.
The authors complemented their detailed, transcriptional profiles by analyzing the gene regulatory programs underlying trophoblast differentiation. They analyzed 34,082 cells from GD7, GD8, and GD9 embryos using Chromium Single Cell ATAC to delineate chromatin accessibility of key developmental markers and transcription factors across rabbit embryogenesis.
They compared transcription factor motif accessibility of known regulators of trophoblast development with their scRNA-seq data. Binding motifs for regulators TEAD4 and CDX2 were accessible during early trophoblast time points but became inaccessible as the trophoblast cells matured. Binding motifs for other regulators opened and closed throughout differentiation as expected based on data from mice and humans, though many were underrepresented in mouse datasets.
But some of their data differed entirely from data obtained from mice. Chromatin accessibility and expression of DXL5/DXL6—genes expressed in humans but not mice—changed during trophoblast development. These genes are major preeclampsia biomarkers—69% of patients with preeclampsia show upregulation of one of these markers (9). The authors cite this finding as evidence that rabbits may be a better model for studying preeclampsia than mice.
The next 100 years
The authors didn’t want to stop there though. They wanted to create a workflow others could use to compare transcriptional profiles across different species. They developed a computational approach to identify similarities between k-nearest neighbor (kNN) graphs. First, the researchers defined neighborhoods of cells in each independent graph. This method avoids the common practice of assigning single cells to discrete clusters based on known cell types and better estimates gene expression changes across developmental stages. Next, the authors calculated an average expression profile for each neighborhood. They compared the average expression profiles of each neighborhood between the two kNN graphs and developed a correlation matrix to measure similarity between neighborhood pairs.
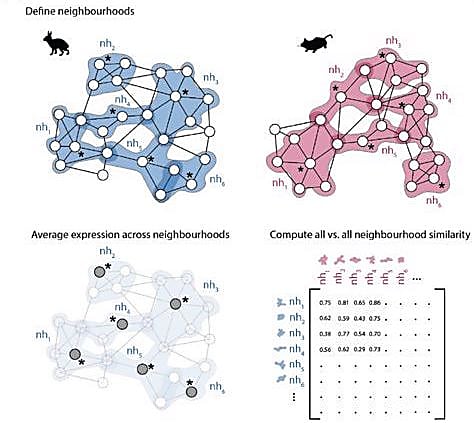
They first used this computational approach to compare scRNA-seq data from rabbits and mice, and, once again, found significant differences in the extraembryonic tissue neighborhoods. They compared paired neighborhoods across the embryonic phases to get a true idea of how the developmental trajectory of extraembryonic tissues differed. The extraembryonic tissue of the gut in rabbit embryos was the least similar to that of mouse embryos. In fact, gut neighborhoods in GD9 rabbit embryos correlated most with gut neighborhoods in E8 mouse embryos, suggesting the developmental timing of early gut development differs between the two species.
The authors then used their newly developed atlas of rabbit embryonic development to gain insight into primate development. They used the automatic annotation method SingleR to label cells in scRNA-seq data from human and macaque embryos based on either rabbit or mouse scRNA-seq data (10). New cells were identified based on the rabbit scRNA-seq annotation in data from both the human and macaque embryos. For example, SingleR identified two types of primordial germ cells (PGCs) that weren’t identified by cluster analysis of the human embryo scRNA-seq data. The mouse-trained annotation model didn’t identify these cells. The authors pointed to a recent study of PGCs that suggested that “key regulators of PGC specification are shared across flat-disc species.”
The researchers provided compelling evidence that comparative biology using scRNA-seq data across a variety of species—especially the rabbit—could be used to provide greater insight into those developmental stages in humans that are hard to catch due to regulatory and technical limitations. Every species has its own value, but, in the end, the authors provide a ton of evidence that this really may be the year of the rabbit.
Explore the study and its resulting atlas for yourself.
And learn how 10x Genomics Chromium Single Cell Gene Expression and ATAC technology can advance your development biology research.
References:
- Subbaraman N. Limit on lab-grown human embryos dropped by stem-cell body. Nature 594: 18–19 (2021). doi: 10.1038/d41586-021-01423-y
- Subbaraman N. Lab-grown structures mimic human embryo's earliest stage yet. Nature 591: 510–511 (2021). doi: 10.1038/d41586-021-00695-8
- Ton M-LN, et al. Rabbit development as a model for single cell comparative genomics. bioRxiv (2022).
- Irie N, et al. Germ cell specification and pluripotency in mammals: a perspective from early embryogenesis. Reprod Med Biol. 13: 203–215 (2014). doi: 10.1007/s12522-014-0184-2
- Van Norman GA. Phase II Trials in Drug Development and Adaptive Trial Design. JACC Basic Transl Sci. 4: 428–437 (2019). doi: 10.1016/j.jacbts.2019.02.005
- Bren L. Frances Oldham Kelsey: FDA Medical Reviewer Leaves Her Mark on History. FDA Consumer Magazine. 35 (2001).
- Theunissen PT, et al. Comparison of rat and rabbit embryo–fetal developmental toxicity data for 379 pharmaceuticals: on the nature and severity of developmental effects. Crit Rev Toxicol. 46: 900–910 (2016). doi: 10.1080/10408444.2016.1224807
- Imaz-Rosshandler I. et al. Tracking early mammalian organogenesis – prediction and validation of differentiation trajectories at whole organism scale. Manuscript in preparation. https://marionilab.github.io/ExtendedMouseAtlas/.
- Zadora J, et al. Disturbed placental imprinting in preeclampsia leads to altered Expression of DLX5, a human-specific early trophoblast marker. Circulation 136: 1824–1839 (2017). doi: 10.1161/CIRCULATIONAHA.117.028110
- https://bioconductor.org/packages/devel/bioc/vignettes/SingleR/inst/doc/SingleR.html
